

I've written about a number of chemistry packages in the past and all of the computational chemistry that you can do in a Linux environment. But, what is fundamental to chemistry? Why, the elements, of course. So in this article, I focus on how you can learn more about the elements that make up everything around you with Kalzium. KDE's Kalzium is kind of like a periodic table on steroids. Not only does it have information on each of the elements, it also has extra functionality to do other types of calculations.
Kalzium should be available within the package repositories for most distributions. In Debian-based distributions, you can install it with the command:
sudo apt-get install kalzium
When you start it, you get a simplified view of the classical periodic table.
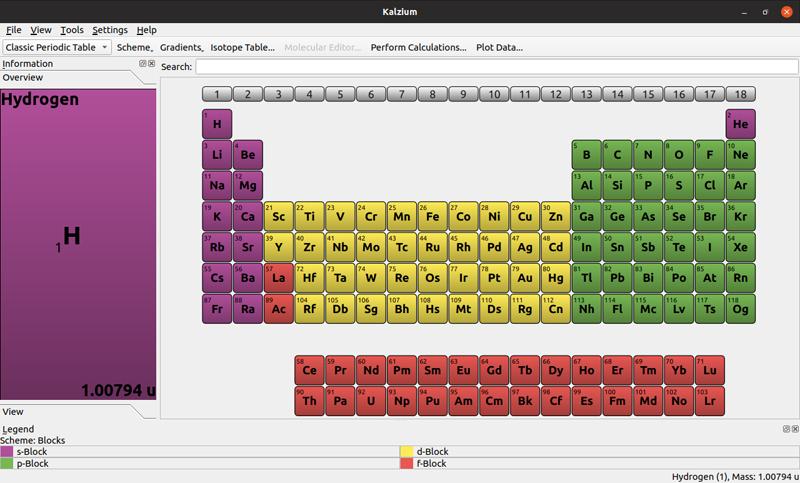
Figure 1. The default view is of the classical ordering of the elements.
You can change this overall view either by clicking the drop-down menu in the top-left side of the window or via the View→Tables menu item. You can select from five different display formats. Clicking one of the elements pops open a new window with detailed information.
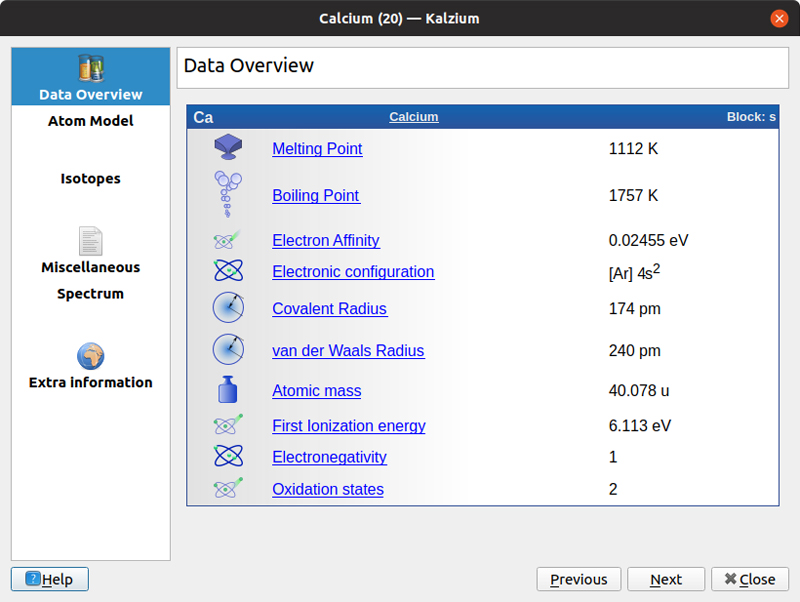
Figure 2. Kalzium provides a large number of details for each element.
The default detail pane is an overview of the various physical characteristics of the given element. This includes items like the melting point, electron affinity or atomic mass. Five other information panes also are available. The atom model provides a graphical representation of the electron orbitals around the nucleus of the given atom. The isotopes pane shows a table of values for each of the known isotopes for the selected element, ordered by neutron number. This includes things like the atomic mass or the half-life for radioactive isotopes. The miscellaneous detail pane includes some of the extra facts and trivia that might be of interest. The spectrum detail pane shows the emission and absorption spectra, both as a graphical display and a table of values. The last detail pane provides a list of external links where you can learn more about the selected element. This includes links to Wikipedia, the Jefferson Lab and the Webelements sites.
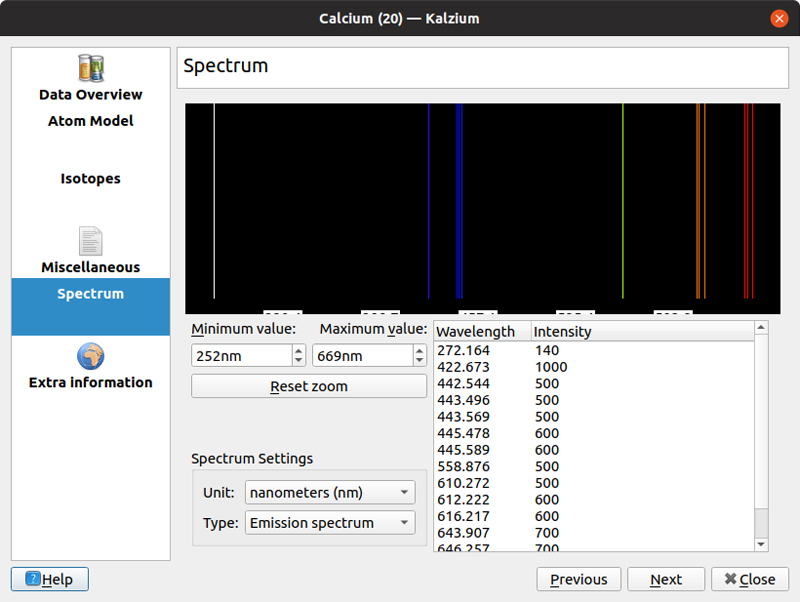
Figure 3. For those elements that are stable enough, you even can see the emission and absorption spectra.
Clicking Tools→Isotope Table pops up a new window with a display of all of the known isotopes.
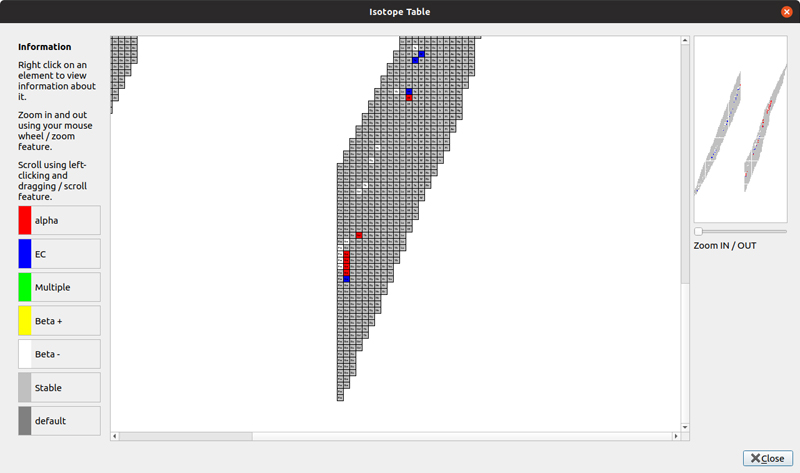
Figure 4. You can get information about all of the known isotopes through an intuitive interface.
Within this window, you can use your mouse to navigate around. The mouse wheel zooms you in and out to a section of the display. You can click and drag the view port to different sections of the isotope display. Once you find the isotope you're interested in, right-click on the given square to see more details on the left-hand side of the window. Here, you can find out about the number of nucleons, the half-life if the isotope is unstable, as well as the relative abundance. For those isotopes that are unstable, the display is color-coded based on the type of radiation that the radioactive element emits.
Clicking Tools→Plot Data opens yet another new window where you can select various characteristics of the elements and plot them. This is helpful for those who process information better visually. You can select from atomic number, atomic mass, electro negativity, melting point, boiling point, atomic radius or covalent radius as the potential data sources. You then can select which elements you want to be plotted.
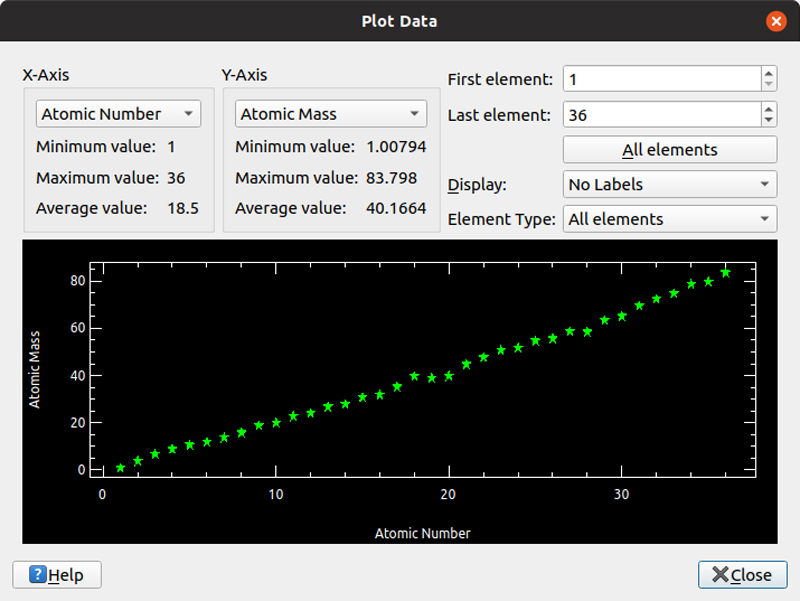
Figure 5. For those who are more visual, you easily can plot relationships between various characteristics of the elements.
Kalzium is also handy for new chemistry students. It includes a glossary and a table of definitions. The glossary explains terms relevant to both knowledge and tools.

Figure 6. If you are new to chemistry, you can use the glossary to learn more about the domain-specific jargon you're likely to encounter.
One slightly odd thing I noticed was that I had to double-click the label in the left-hand pane in order to have the contents actually be displayed in the right-hand pane. If you are particularly new to science, you may not know Greek symbols or size prefixes for numbers. If that's the case, click Tools→Tables. This way, you easily can get reminders about what a hectometer, for instance, works out to be.
The last section I want to look at is the group of calculators Kalzium provides. Click Tools→Perform Calculations to open a new window. You can do calculations on molecular mass, concentration, nuclear information, gas information, titration or equation balancing. In the molecular mass calculator, you can enter a chemical formula and have it calculate the total molecular mass.
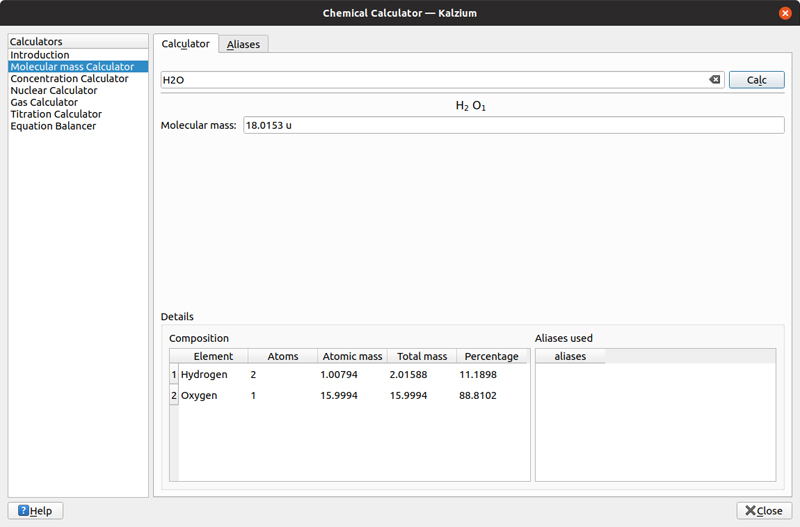
Figure 7. Kalzium can figure out the mass of a given molecular formula.
In some cases, you'll have larger units of molecules, especially in organic chemistry. In those situations, you can create aliases of these units, such as alcohols or benzene rings, making it easier to create your molecular formula. The second calculator figures out concentrations, in moles, for given amounts of solute and solvent. Here, you can adjust the various amounts of both the solvent and the solute to figure out what concentration you end up with.
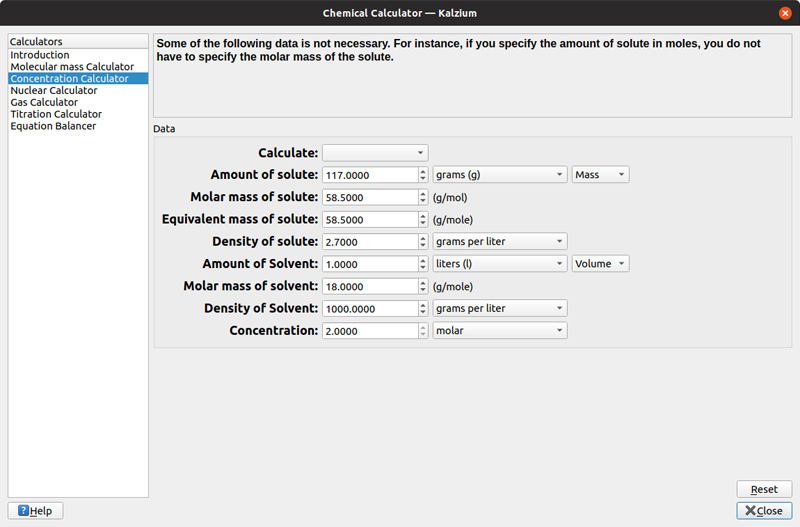
Figure 8. Kalzium provides a calculator to help you figure out the molarity for a given solution.
The third calculator is the nuclear calculator. With this, you can select an isotope, with its isotope mass and its half-life. You then can solve for either an initial amount, a final amount or the time to get between an initial and a final amount.
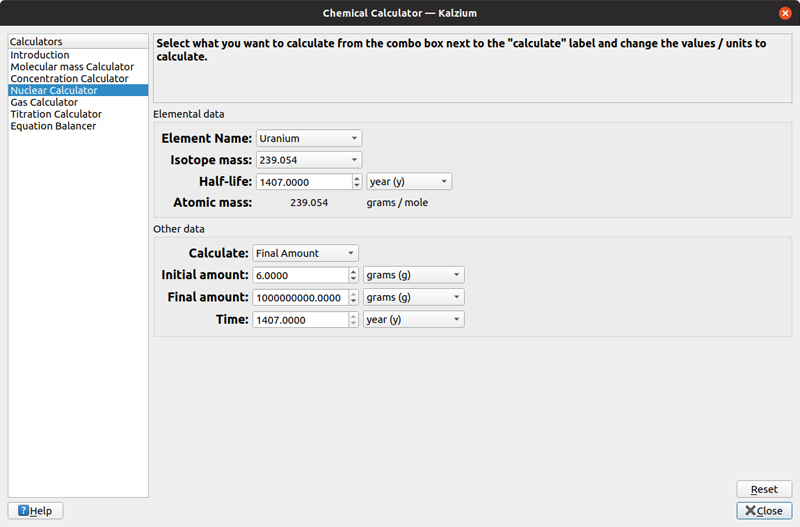
Figure 9. You can figure out how long a given piece of radioactive material will hang around.
The fourth calculator takes the gas law and dynamically calculates the other values when you change one of the values. Kalzium lets you play with the temperature, pressure and volume, given the set of gas parameters molar mass and number of moles. This can be useful when you first start to study how gases respond to temperature and pressure changes.
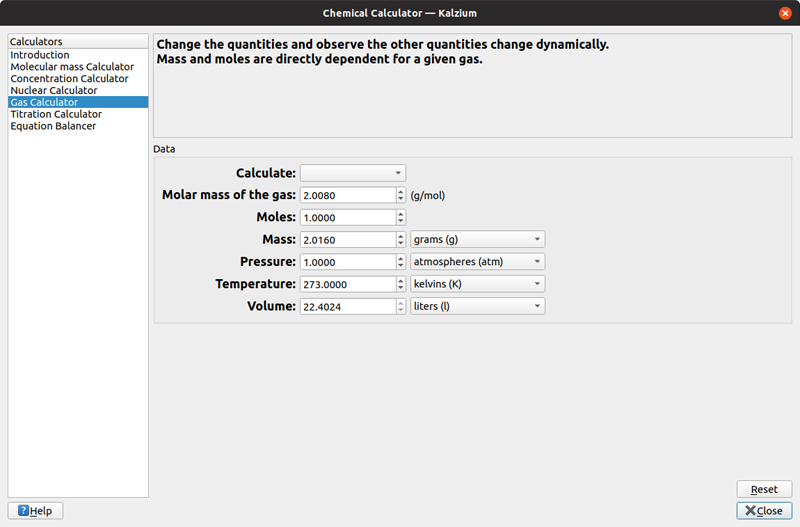
Figure 10. Kalzium provides a calculator that simplifies the calculations defined by the gas law.
The fifth calculator provides titration calculations. You can enter experimental values of pH and volume, and then Kalzium will try to plot the inputs and figure out a theoretical equation.
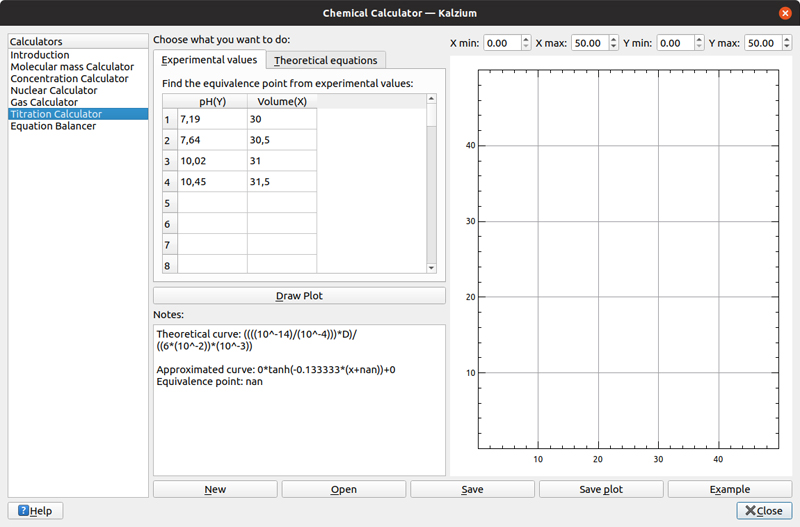
Figure 11. If you are studying acids and bases, Kalzium provides a titration calculator.
The last calculator is the equation-solver. You can provide a chemical equation, where you enter unknowns as lower-case letters placed in front of the element symbols. When you click the calculate button, Kalzium will figure out what value those lower-case variables should have.
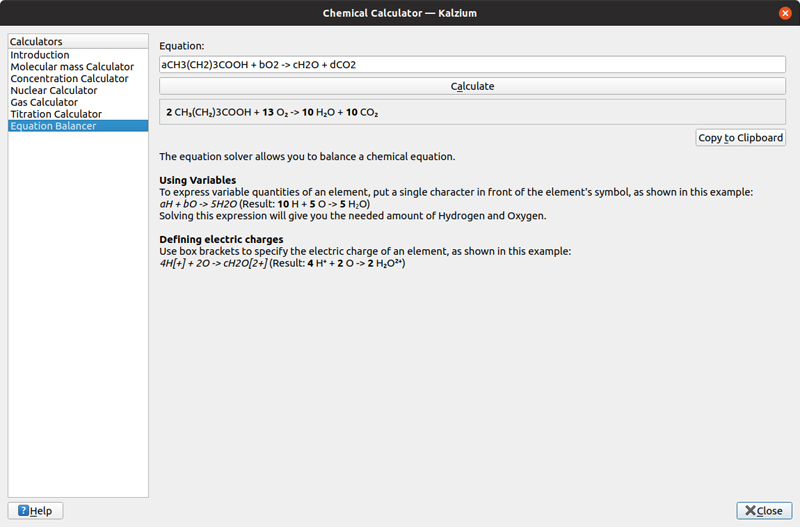
Figure 12. Kalzium can take a given chemical equation and figure out missing values to make the equation balance.
I hope Kalzium comes in handy for you. I think it would be especially helpful for students just getting started in chemistry.
—Joey Bernard